WHY GETTING VACCINATED AGAINST COVID-19?
Covid-19 vaccination greatly reduces the chance of you suffering from Covid-19 disease. No vaccine is 100% effective, however numerous studies have shown 75-85% efficacy of the AstraZeneca Covid-19 vaccine against symptomatic Covid-19 and an impressive 100% efficacy against severe or critical disease and hospitalisations. This means that the few vaccinated people who do get infected have essentially mild to moderate cases of Covid-19 and that your risk of hospitalisation and death because of Covid-19 is nearly eliminated once you are fully vaccinated.
Also, vaccinated people who might be infected with the coronavirus have fewer virus particles in their nose and mouth and are less likely to spread it to others. This reduced transmission is important as getting vaccinated now not only protects you, but also limits spreading the virus to loved ones, friends and other people.
Reduced transmission is such that, on 13 May 2021, the US Centers for Disease Control and Prevention (CDC) have released an updated guidance saying that fully vaccinated people (2 weeks after their second dose) no longer need to wear a face mask or respect physical distancing in most settings, whether outdoors or indoors.
In addition, recent studies have shown that, after 2 doses, the AstraZeneca Covid-19 vaccine is highly effective at protecting people against specific coronavirus strains (called “variants of concern”) such as the Indian, the Brazilian, the South African and the Kent variants.
WHAT IS ASTRAZENECA COVID-19 VACCINE?
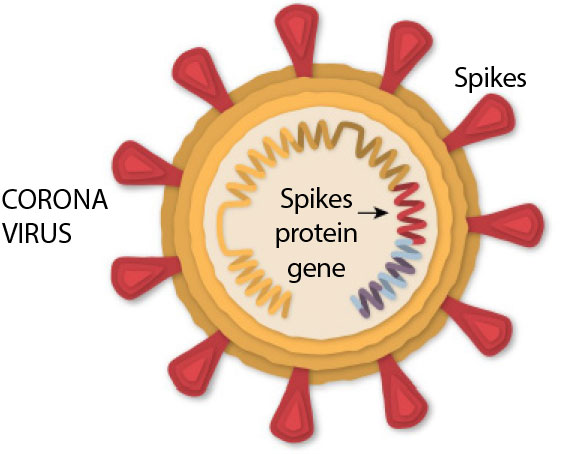
Covid-19 disease is caused by the SARS-CoV-2 virus. AstraZeneca Covid-19 vaccine is made up of another virus (of the adenovirus family) that has been modified to contain the gene for making the Covid-19 virus spike protein. This is a protein on the surface of the virus which the virus needs to enter the body’s cells.
HOW DOES ASTRAZENECA COVID-19 VACCINE WORK?
Once it has been given, the vaccine delivers the spike gene into cells in the body. The cells will use the gene to produce the spike protein. The person’s immune system will then recognise this protein as foreign and produce antibodies (by the “B cells”) and activate specific white blood cells (called “T cells”) to attack it.
If, later on, the person comes into contact with SARS-CoV-2 virus, their immune system will recognise it and be ready to defend the body against it.
AstraZeneca Covid-19 vaccine does not contain the Covid-19 virus itself and cannot cause Covid-19 disease. The adenovirus in the vaccine cannot reproduce and cannot cause disease.
CAN THE COVID-19 VACCINE CAUSE A POSITIVE TEST RESULT FOR THE DISEASE, SUCH AS FOR A PCR OR ANTIGEN TEST?
No, the Covid-19 vaccine will not cause a positive test result for a Covid-19 PCR or antigen laboratory test. This is because these tests check for active disease and not whether an individual is immune or not. However, because the Covid-19 vaccine prompts an immune response, vaccinated people usually test positive in an antibody (serology) test that measures Covid-19 immunity in an individual.
HOW IS ASTRAZENECA COVID-19 VACCINE ADMINISTERED?
The AstraZeneca Covid-19 vaccine is injected into a muscle (usually in the upper arm). The vaccine will be given in 2 doses. You will be advised when to return for your second dose. The second dose can be given between 4 and 12 weeks after the first dose.
Post-vaccination observation times:
- 30 minutes: persons with a history of an immediate allergic reaction of any severity to a vaccine or injectable therapy or a history of anaphylaxis due to any cause;
- 15 minutes: all other persons.
HOW LONG DOES PROTECTION FROM ASTRAZENECA COVID-19 VACCINE LAST?
Covid-19 vaccination offers good protection within 2 to 3 weeks of the first dose. It takes 2 weeks after getting the second dose to have the best protection, therefore it’s essential to get both doses to protect yourself against Covid-19.
It is not currently known how long the protection given by AstraZeneca Covid-19 vaccine lasts, however experts believe that immunity from Covid-19 vaccines will last longer than six months and most probably at least longer than one year.
CAN CHILDREN BE VACCINATED WITH ASTRAZENECA COVID-19 VACCINE?
AstraZeneca Covid-19 vaccine is currently only authorised for people aged 18 years and older. Trials are being conducted involving children, and other Covid-19 vaccines are already authorised in several countries for children between 12 and 17-year old.
CAN IMMUNOCOMPROMISED PEOPLE BE VACCINATED WITH ASTRAZENECA COVID-19 VACCINE?
Although immunocompromised people (people with weakened immune systems) may not respond as well to the vaccine, there are no particular safety concerns. Immunocompromised people should be vaccinated as they may be at higher risk from Covid-19.
CAN PREGNANT OR BREAST-FEEDING WOMEN BE VACCINATED WITH ASTRAZENECA COVID-19 VACCINE?
Pregnant women when vaccinated create antibodies to the virus and pass those to their unborn baby through the placenta.
Pregnant people are at increased risk for severe illness from Covid-19. Women with Covid-19 disease are also 2 to 3 times more likely to have their babies early than women without Covid-19 and might be at increased risk of other adverse pregnancy outcomes. Pregnant women with underlying clinical conditions are at even higher risk of suffering serious complications from Covid-19.
For these reasons, many countries including the US, the UK, Israel and France recommend that pregnant people get vaccinated and are to be treated as priority cases. The vaccine can be given at any stage during pregnancy. There is no reason to delay a pregnancy after Covid-19 vaccination.
Covid-19 vaccines are thought not to be a risk to lactating people or their breastfeeding babies. Actually mothers were shown to pass antibodies to their newborns through breast milk. Therefore, lactating people can receive a Covid-19 vaccine.
CAN PEOPLE VACCINATED RECENTLY WITH ANOTHER VACCINE RECEIVE ASTRAZENECA COVID-19 VACCINE?
Covid-19 vaccines and other vaccines may be administered without regard to timing. This includes simultaneous administration of Covid-19 vaccine and other vaccines on the same day, as well as co-administration within 14 days.
DO YOU HAVE A BLEEDING DISORDER OR ARE YOU TAKING A BLOOD THINNER?
As with all vaccines, Covid-19 vaccine may be given to these patients, if a doctor determines that the patient’s bleeding risk is low enough and the vaccine can be administered intramuscularly with reasonable safety. The following technique for intramuscular vaccination in patients with bleeding disorders or taking blood thinners is recommended: a fine-gauge needle (23-gauge or smaller calibre) should be used for the vaccination, followed by firm pressure on the site, without rubbing, for at least 2 minutes
ARE THERE ANY REASONS YOU SHOULD NOT GET THE VACCINE?
There are very few people who cannot get the Covid-19 vaccine.
The vaccine should not be given to:
- People who have had a confirmed anaphylactic reaction to any of the ingredients of the vaccine;
- those who have had a confirmed anaphylactic reaction to a previous dose of the same Covid-19 vaccine.
People with a history of serious allergic reaction to food, an identified drug or vaccine, or an insect sting can get any Covid-19 vaccine, as long as they are not known to be allergic to any component of the Covid-19 vaccine. It’s important that you tell the person giving you your vaccine if you have ever had a serious allergic reaction (anaphylaxis).
Are you feeling sick today?
There is no evidence that acute illness reduces vaccine efficacy or increases vaccine adverse events. However, as a precaution with moderate or severe acute illness, all vaccines should be delayed until the illness has improved. Mild illnesses (e.g., upper respiratory infections, diarrhoea) are NOT contraindications to vaccination. Do not withhold vaccination if a person is taking antibiotics.
Have you received another vaccine in the last 14 days?
The Covid-19 vaccine should be administered alone, with a minimum interval of 14 days before or after administration of other vaccines.
WHAT ARE THE RISKS ASSOCIATED WITH ASTRAZENECA COVID-19 VACCINE?
Like all medicines, vaccines can cause side effects. So far, millions of people have been given the AstraZeneca Covid-19 vaccine and reports of serious side effects, such as allergic reactions and blood clots, have been very rare. No long-term complications have been reported. Most of the AstraZeneca Covid-19 vaccine side effects are mild and short-term (a few days), and not everyone gets them (about 10-20% of people), they include:
- A sore arm where the needle went in;
- Feeling tired;
- A headache;
- Feeling achy;
- Nausea;
- Some people will experience mild flu-like symptoms;
- Very few people get a high temperature or feel hot or shivery 1 or 2 days after vaccination;
- An uncommon side effect is swollen glands in the armpit or neck on the same side as the arm where you had the vaccine. This can last for around 10 days, but if it lasts longer see your doctor.
Allergic reactions
Allergic reactions (hypersensitivity) have been seen in people receiving the vaccine. Cases of anaphylaxis (severe allergic reaction) are rare. If you do have a reaction to the vaccine, it usually happens in minutes. Signs of an allergic reaction may include itchy skin rash, shortness of breath and swelling of the face or tongue.
As for all vaccines, AstraZeneca Covid-19 vaccine should be given under medical supervision, with the appropriate medical treatment available in case of allergic reactions. Staff giving the vaccine are trained to deal with allergic reactions and treat them immediately.
Blood clots
There have been very rare cases of blood clots (thrombosis), approximately 1 incident for 200,000 to 400,000 people vaccinated, so an incidence of less than 0,001% that should be compared to the 0,5% to 1% risk of death for people who contract Covid-19. These blood clots can be venous or arterial, particularly at unusual sites such as in the brain or abdomen, accompanied by low levels of blood platelets, and occur 5 days to 4 weeks after vaccination.
ARE THE SIDE EFFECTS DIFFERENT FOR EACH DOSE?
Side effects can occur after the first and/or the second dose. Even if you did have side effects after the first dose, you still need to have the second dose (except if you had a severe allergic reaction after the first dose).
WHAT SHOULD YOU DO IF YOU ARE EXPERIENCING SIDE EFFECTS?
If you are experiencing side effects, rest until you feel better. You can take pain killers, such as paracetamol, if you need to.
You should seek medical advice:
- If your symptoms seem to get worse or if you are concerned;
- If you have swollen glands for more than 10 days;
- In case of abnormal, persistent symptoms, such as high fever for more than 4 days.
If you experience any of the following from around 4 days to 4 weeks after vaccination, you should seek medical advice urgently:
- Bruising somewhere other than where you had your vaccination;
- Shortness of breath, chest pain, leg swelling or persistent abdominal pain;
- A new, severe headache which is not helped by usual painkillers or is getting worse;
- A headache which seems worse when lying down or bending over, or is accompanied by blurred vision, nausea and vomiting, difficulty with your speech, weakness, drowsiness or seizures.
You can contact, at any time, FV Hospital Accident & Emergency Department at
References
- Everything you need to know about COVID-19 vaccines. The Pharmaceutical Journal, May 2021; Online: DOI:10.1211/PJ.2021.1.71237
- Vaccines for COVID-19. Centers for Disease Control and Prevention (CDC). https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
- Coronavirus (COVID-19) vaccine. NHS. https://www.nhs.uk/conditions/coronavirus-covid-19/coronavirus-vaccination/coronavirus-vaccine/
- Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. The Lancet Volume 397, Issue 10282, P1351-1362, April 10, 2021
- Important information about pregnancy and breastfeeding. NHS Scotland. nhsinform.scot/covid19vaccinepregnancy
- COVID-19 vaccines and pregnancy. Royal College of Obstetricians and Gynaecologists. https://www.rcog.org.uk/covid-vaccine
- COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html

 Vi
Vi