By Jonathan Corum and Carl Zimmer
Updated Jan. 21, 2021
Moderna, a Massachusetts-based vaccine developer, partnered with the National Institutes of Health to develop and test a coronavirus vaccine known as mRNA-1273. A clinical trial demonstrated that the vaccine has an efficacy rate of 94.1 percent in preventing Covid-19.
A Piece of the Coronavirus
The SARS-CoV-2 virus is studded with proteins that it uses to enter human cells. These so-called spike proteins make a tempting target for potential vaccines and treatments.
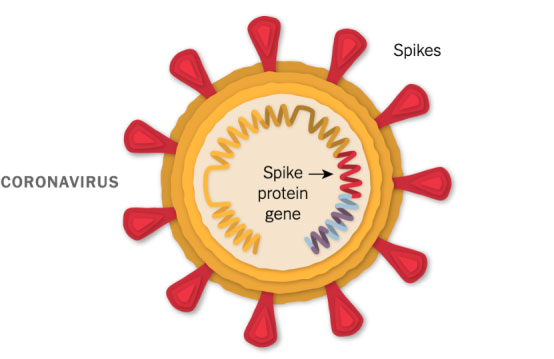
Like the Pfizer-BioNTech vaccine, Moderna’s vaccine is based on the virus’s genetic instructions for building the spike protein.
mRNA Inside an Oily Shell
The vaccine uses messenger RNA, genetic material that our cells read to make proteins. The molecule — called mRNA for short — is fragile and would be chopped to pieces by our natural enzymes if it were injected directly into the body. To protect the vaccine, Moderna wraps the mRNA in oily bubbles made of lipid nanoparticles.
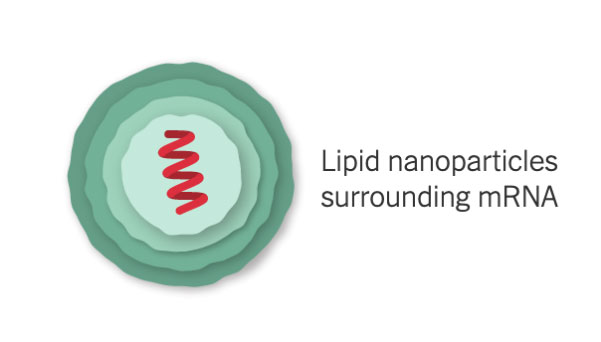
Because of their fragility, the mRNA molecules will quickly fall apart at room temperature. Moderna’s vaccine will need to be refrigerated, and should be stable for up to six months when shipped and stored at –4°F (–20°C).
Entering a Cell
After injection, the vaccine particles bump into cells and fuse to them, releasing mRNA. The cell’s molecules read its sequence and build spike proteins. The mRNA from the vaccine is eventually destroyed by the cell, leaving no permanent trace.
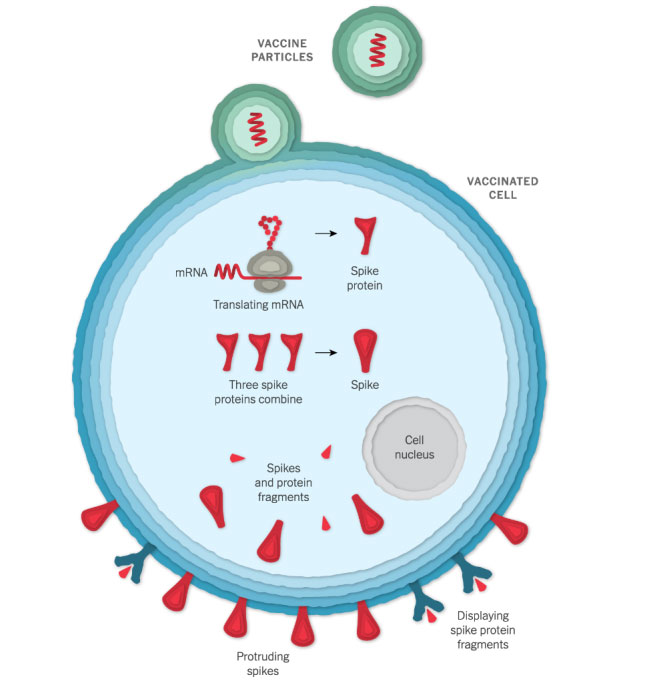
Some of the spike proteins form spikes that migrate to the surface of the cell and stick out their tips. The vaccinated cells also break up some of the proteins into fragments, which they present on their surface. These protruding spikes and spike protein fragments can then be recognized by the immune system.
Spotting the Intruder
When a vaccinated cell dies, the debris will contain many spike proteins and protein fragments, which can then be taken up by a type of immune cell called an antigen-presenting cell.
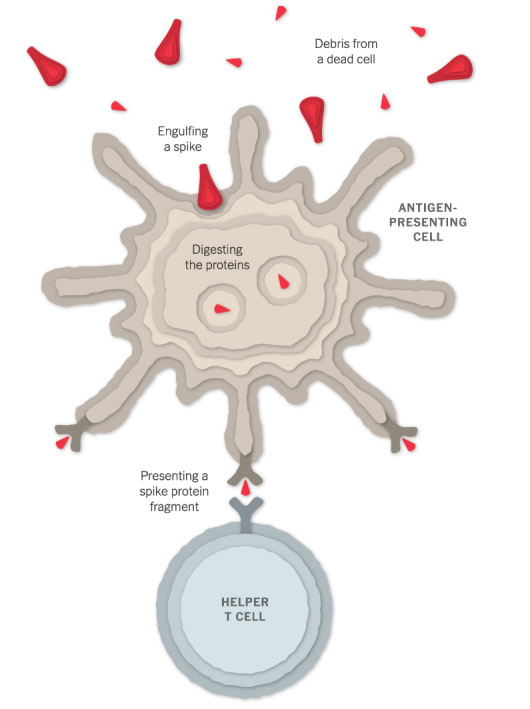
The cell presents fragments of the spike protein on its surface. When other cells called helper T cells detect these fragments, the helper T cells can raise the alarm and help marshal other immune cells to fight the infection.
Making Antibodies
Other immune cells, called B cells, may bump into the coronavirus spikes on the surface of vaccinated cells, or free-floating spike protein fragments. A few of the B cells may be able to lock onto the spike proteins. If these B cells are then activated by helper T cells, they will start to proliferate and pour out antibodies that target the spike protein.
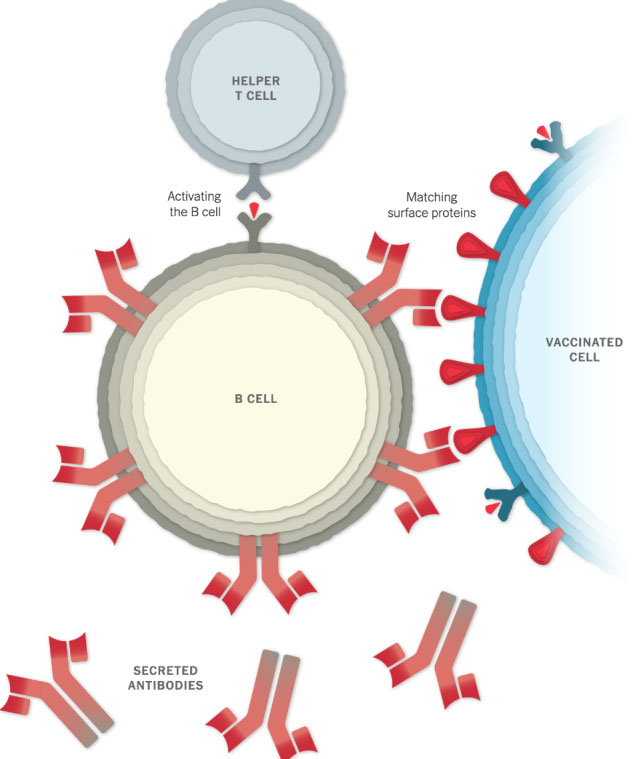
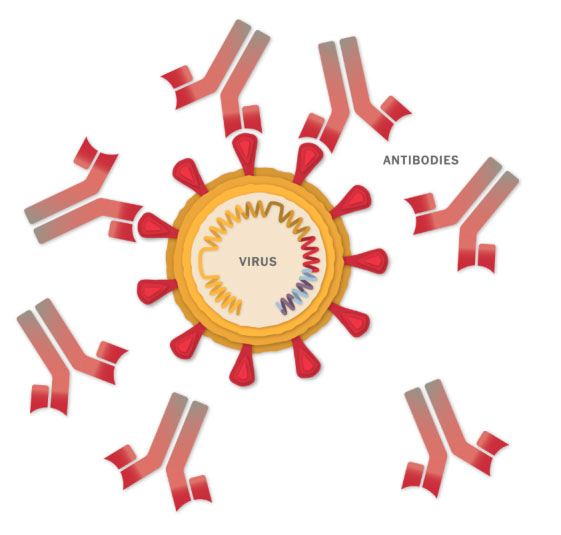
Stopping the Virus
The antibodies can latch onto coronavirus spikes, mark the virus for destruction and prevent infection by blocking the spikes from attaching to other cells.
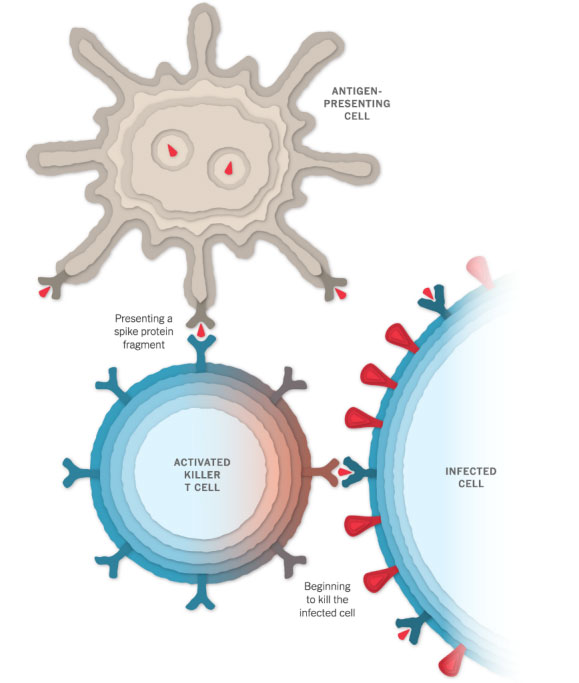
Killing Infected Cells
The antigen-presenting cells can also activate another type of immune cell called a killer T cell to seek out and destroy any coronavirus-infected cells that display the spike protein fragments on their surfaces.
Remembering the Virus
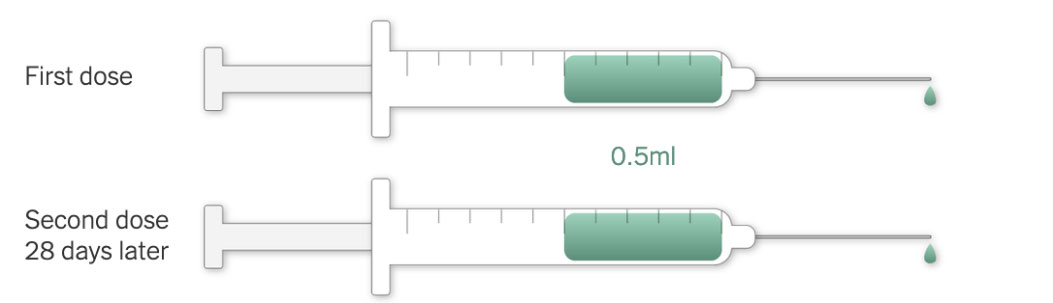
Moderna’s vaccine requires two injections, given 28 days apart, to prime the immune system well enough to fight off the coronavirus. But because the vaccine is so new, researchers don’t know how long its protection might last.
It’s possible that in the months after vaccination, the number of antibodies and killer T cells will drop. But the immune system also contains special cells called memory B cells and memory T cells that might retain information about the coronavirus for years or even decades.
An early study found that Moderna’s vaccine provides protection for at least three months.
Preparation and Injection
Each vial of the vaccine contains 10 doses of 0.5 milliliters. The vials need to be warmed to room temperature before injection. No dilution with saline is required.
Vaccine Timeline
January 2020 Moderna begins work on a coronavirus vaccine.
March 16 Moderna scientists are the first to put a Covid-19 vaccine into human trials.
April 16 Moderna announces that the United States government will provide $483 million in support for the design and testing of Moderna’s vaccine. Researchers at the National Institutes of Health will oversee much of the research, including the clinical trials.
July 27 After initial studies yield promising results, Moderna and the N.I.H. begin Phase 3 testing on 30,000 volunteers across the United States. A quarter of the participants are 65 years or older.
July 28 Moderna finds that the vaccine protects monkeys from the coronavirus.
Aug. 11 The United States government awards the company an additional $1.5 billion in exchange for 100 million doses, if the vaccine is authorized by the Food and Drug Administration.
Nov. 16 Moderna announces preliminary data from its Phase 3 trial. Researchers estimate that the vaccine has an efficacy rate of 94.1 percent, far higher than experts had expected when vaccine testing began.
Nov. 30 Moderna applies for emergency use authorization from the F.D.A.
Dec. 2 Moderna registers a trial to test the vaccine on children between 12 and 18 years of age.
Dec. 18 The F.D.A. authorizes the Moderna vaccine for emergency use. The first injections of its vaccine could start on Dec. 21.
Dec. 23 Canada authorizes the vaccine.
Dec. 31 The company expects to produce 20 million doses by the end of this year, and up to a billion doses in 2021. Each vaccinated person will require two doses.
Jan. 4 Israel authorizes the vaccine for emergency use.
Spring 2021 Vaccines by Moderna and Pfizer-BioNTech are expected to reach large-scale distribution in the spring.
Sources: National Center for Biotechnology Information; Nature; Florian Krammer, Icahn School of Medicine at Mount Sinai.

 Vi
Vi